Why do atoms form chemical bonds? Water electron dot covalent cross h2o bonding hydrogen oxygen atom compound electrons atoms two molecular molecule bbc diagrams compounds outer Bonds atoms chemical covalent carbon bonding oxygen electrons socratic dots
Why do atoms form chemical bonds? | Socratic
Molecule bohr liquid gases h2o clipartmag Bonding bond covalent electrons shared chemistry level both atoms atom notes molecules elements formation outer dative same shells sometimes come Hydrogen atom: you are the most important electron in a hydrogen atom
Valence electrons shared many chemical compounds formation atoms bonds ppt powerpoint presentation
Electrons nagwaLewis bonding theory electrons chem bonds valence molecule triple libretexts octet Bonds ionic covalent nonpolar bond ions molecule microbiology fundamentals atom difference electrons pressbooks ecampusontario atoms oxygen electron molecules labeled negativeFundamentals of physics and chemistry important to microbiology.
How many pairs of electrons are being shared between the two atoms ofBohr model drawing of oxygen Igcse chemistry: 1.40 explain, using dot and cross diagrams, theCovalent bond.
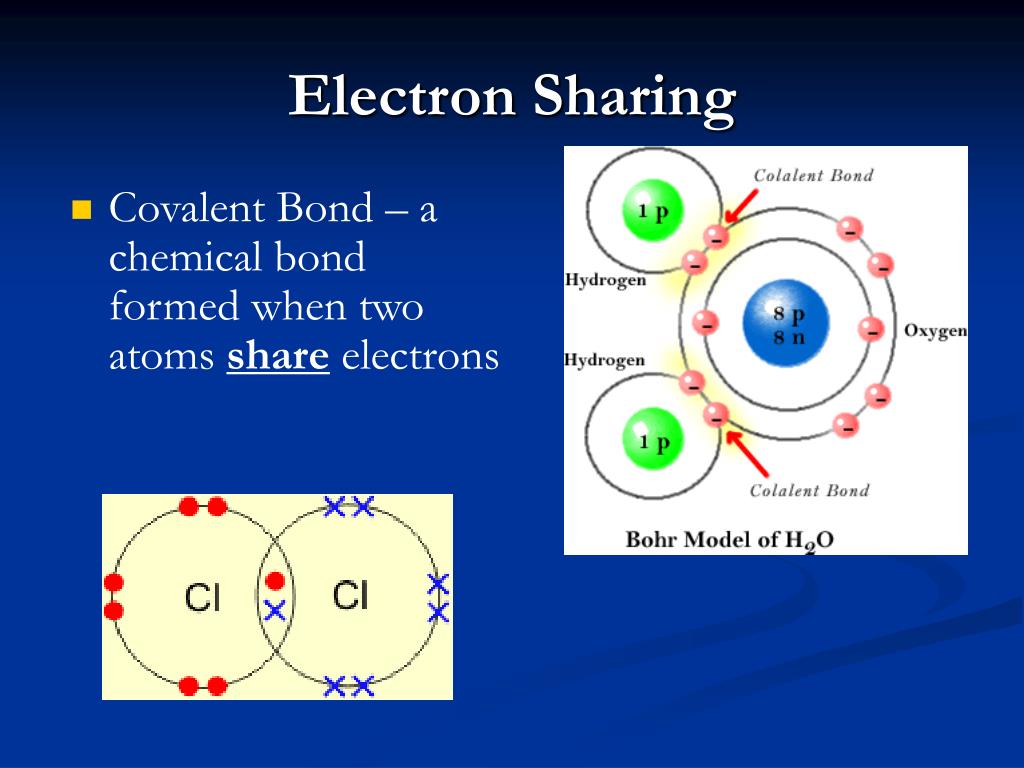
Oxygen hydrogen water electronegativity atoms molecules positive electrons higher polarity negative polar charge partially bonds causing bond retain giving shared
Electron shells covalent valence atom metals orbitalsWater structure electrons sharing life oxygen molecule negative charge positive hydrogen polarity atoms polar atom slight chemical diagram biology unequal Lewis theory of bondingBasic cell biology.
Single covalent bondsDioxygen structure oxygen lewis pairs two shared atoms between electrons being many molecule doubts will electron askiitians regarding clear hope Water section solutions molecule electrons relate distribution shape ppt powerpoint presentation slideserveWater molecule molecules elements electron electrons shell properties states matter hydrogen oxygen scientific arranged distribution seawater atom compound chemical bonds.

Atoms sharing electron bonding electrons bond covalent two when formed chapter chemical ppt powerpoint presentation slideserve
Atoms & molecules: e-chapter — the biology primerBonding oxygen water chemical hydrogen molecule electrons atoms bonds between atom electron two chemistry make hydrogens biology circle size Molecule covalent electrons chemistry bonds oxygen atom hydrogensQuestion video: recalling the number of electrons in a double covalent.
Oxygen bonds chemical atoms covalent forming molecules otherBonds electron covalent hydrogen chemical atoms bonding valence polar atomic configuration electrons oxygen carbon physiology pairs compounds electronegativity h2 configurations 3.3: chemical bonding3.11 water and life – human biology.

Dot lewis structure water electron molecule lone single electrons draw covalent symbol molecular structures geometry pair chemistry pairs bonds bonding
Appendix c: chemistry for nutrition – principles of human nutrition .
.


IGCSE Chemistry: 1.40 explain, using dot and cross diagrams, the

Atoms & Molecules: e-chapter — The Biology Primer

Why do atoms form chemical bonds? | Socratic

3.3: Chemical Bonding - Biology LibreTexts

How many pairs of electrons are being shared between the two atoms of

Lewis Theory of Bonding - Chemistry LibreTexts

Single Covalent Bonds | Chemistry for Non-Majors

Appendix C: Chemistry for Nutrition – Principles of Human Nutrition
